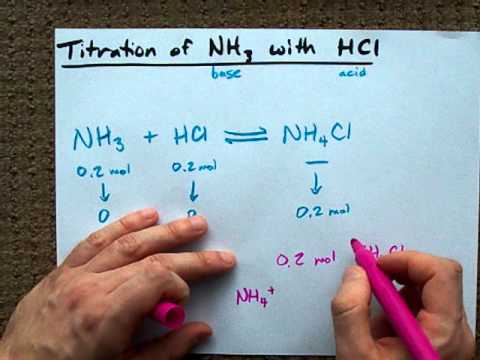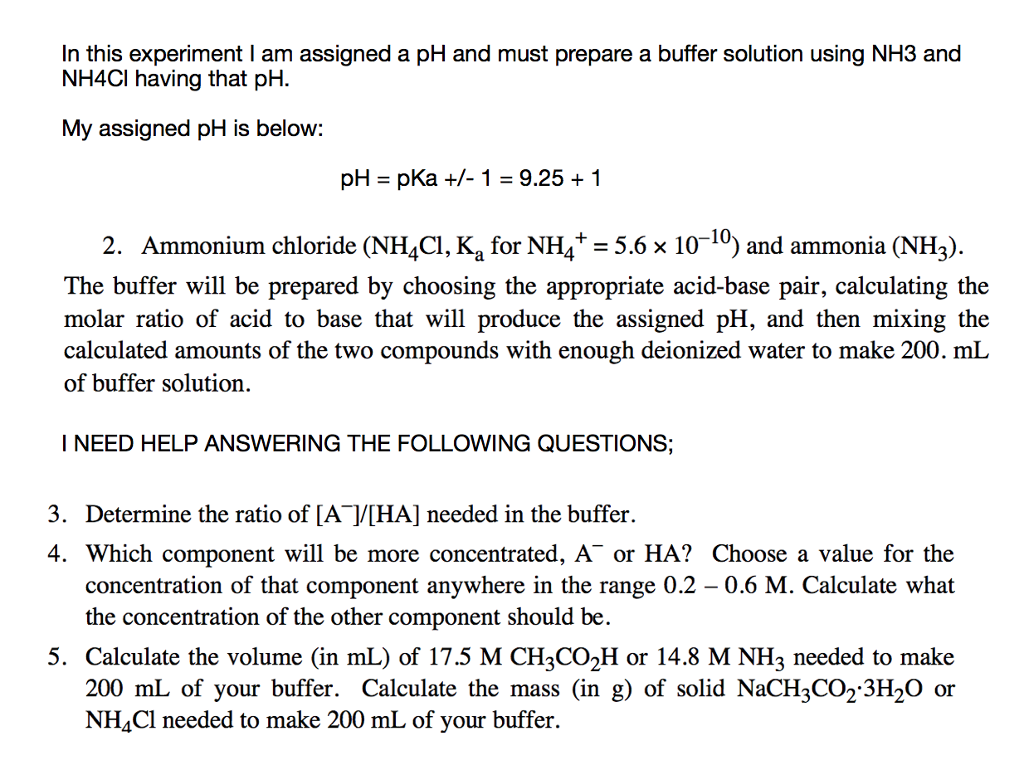
OneClass: Calculate the pH of the 0.20 M NH3/0.20 M NH4Cl buffer. What is the pH of the buffer after ...

A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia). NH3(aq) + H2O(l) = NH4+ (aq) + OH-(aq) What happens

Q.11 The pH of buffer of NH4OH + NH4Cl - type is given by -| NEET PRACTICE TEST -2| VISHAL ACADEMY - YouTube

ka = 7 x 10.) 31. An ammonia-ammonium chloride buffer has a pH value of 9 with (NH3) = 0.25. By how much the pH will change if 75 mL of 0.1

OneClass: Calculate the pH of a .20M NH3/.20M NH4Cl buffer after the addition of 20.0mL of 0.10M HCl ...

OneClass: does the addition of HCl to a NH4Cl/ NH3 buffer solution produce the common ion effect? why...

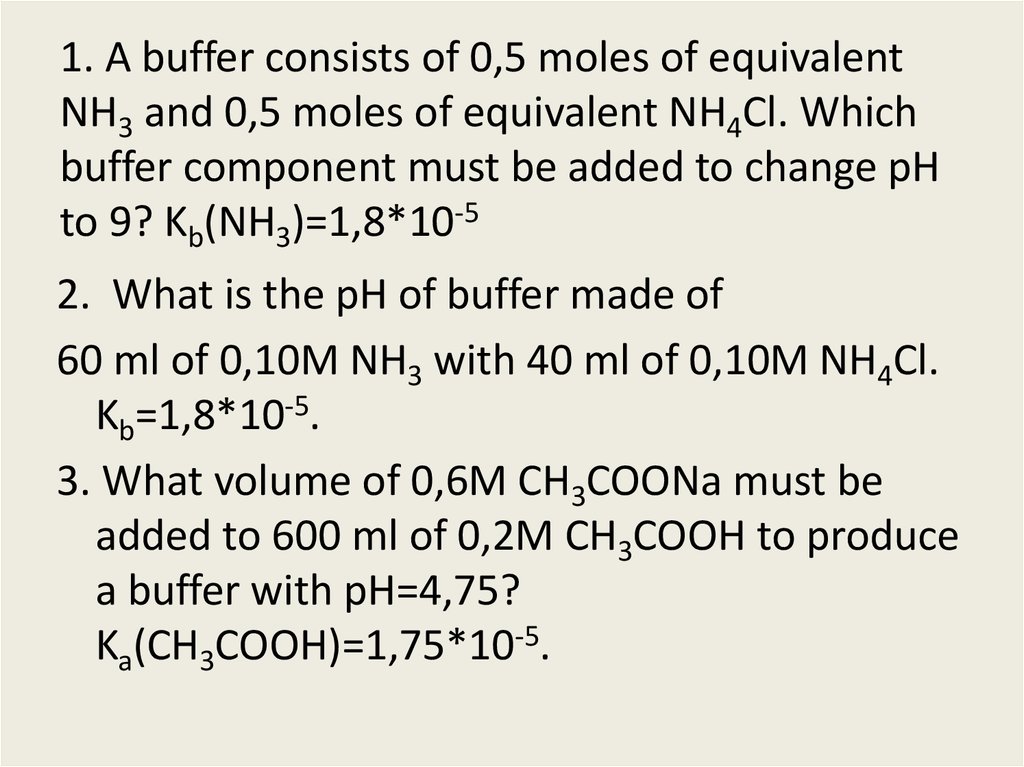
OneClass: Consider a buffer solution that is 0.50 M in NH3 and 0.20 M in NH4Cl. For ammonia, pKb=4.75...

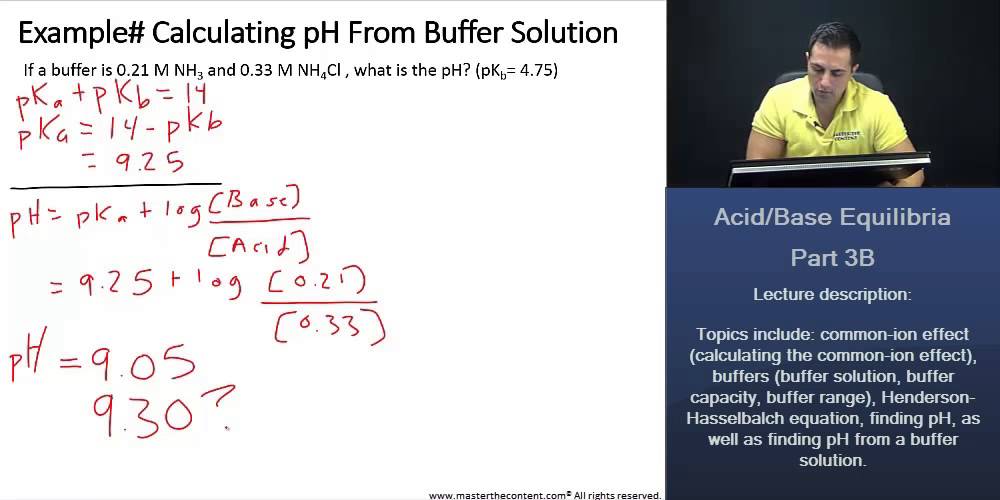
pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube